Kevin Esvelt in 2015.Rodrique Ngowi/AP
Not long ago, Bill Gates, whose family foundation has spent billions of dollars battling diseases around the globe, noted in his blog that the deadliest animals on the planet are not sharks or snakes or even humans, but mosquitoes. Technically, the bloodsuckers merely host our most dangerous creatures. Anopheles mosquitoes can incubate the protozoae responsible for malaria—a stubborn plague that inspired the DDT treatment of millions of US homes and the literal draining of American swamps during the 1940s to shrink the insects’ breeding grounds. Malaria is now rare in the United States, but it infected an estimated 212 million people around the world in 2015, killing 429,000—mostly kids under five.
Dengue, a virus that infects up to 100 million people worldwide each year, is spread largely by Aedes aegypti mosquitoes, which thrive along our Gulf Coast and also are capable of transmitting the related viruses Zika, chikungunya, and yellow fever. Of the millions infected, roughly 500,000 dengue victims develop an excruciatingly painful “break-bone fever”—according to Laurie Garrett’s The Coming Plague, “dengue” derives from the Swahili phrase ki denga pepo, “it is a sudden overtaking by a spirit”—and tens of thousands die.
West Nile virus, spread by Culex mosquitoes, has killed more than 2,000 Americans since 1999, primarily in California, Colorado, and Texas. Our latest headache, Zika, produces ghastly brain defects in the infants of infected mothers and neurological symptoms in some adults. Puerto Rico has been ravaged by more than 35,000 mosquito-borne Zika cases since 2015, not to mention periodic dengue outbreaks that afflict tens of thousands of people.
What if we could make all of this go away?
We do, in fact, have a weapon that could end the mosquito’s reign of terror. It’s called “gene drive.” Its implications are thrilling, and also kind of terrifying.
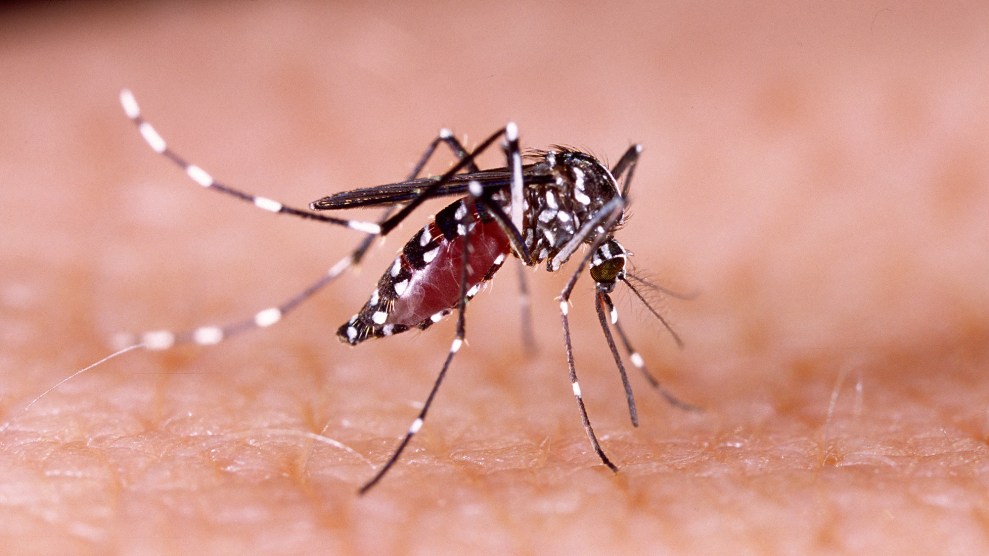
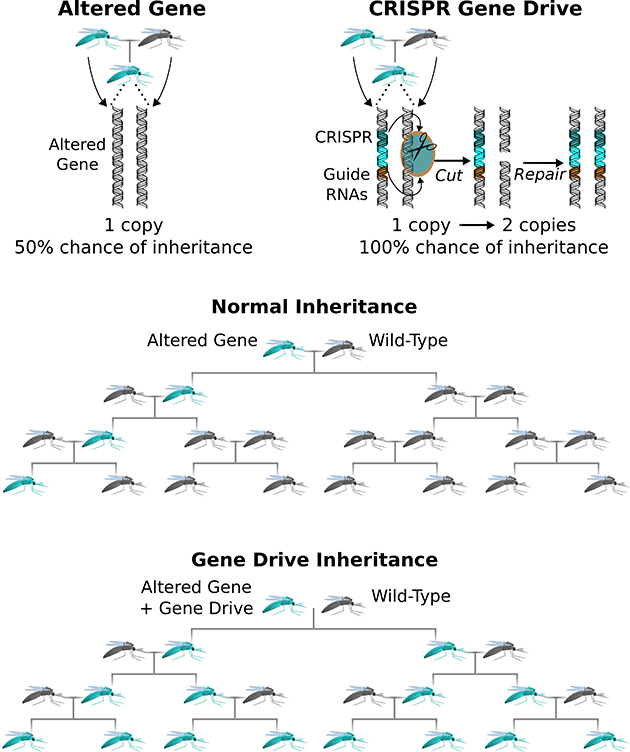
Evolution is a numbers game. Say you were able to create a modified gene in the lab and insert it into into the genome of an early-stage animal embryo. By the rules of inheritance, the anomaly you introduced would be passed along to roughly half of that creature’s offspring. Assuming the new gene you inserted didn’t offer any survival advantage (or disadvantage), it would be inherited by about a quarter of the subsequent generation and then an eighth and a sixteenth, and so on—until it became the genetic equivalent of radio static.
Gene drive upends that calculus. This powerful new technique, lab-tested so far in yeast, fruit flies, and mosquitoes, guarantees that a modified genetic trait is inherited by virtually all of a creature’s offspring and all of their offspring. After a while, every creature in the population carries the modification.
This wouldn’t work in people, thankfully—a short reproductive cycle and plenty of offspring are required for gene drives to spread themselves effectively. But one could build, for instance, a drive targeting Aedes mosquitoes that leaves their offspring unable to reproduce, or a drive that renders Anopheles mosquitoes incapable of spreading malaria. You could design a gene drive to control a stubborn crop pest or to render white-footed mice unable to act as a vessel through which ticks pick up and spread Lyme disease.
If used with care, gene drive could save millions of lives and billions of dollars. It could reduce pesticide use, help weed out nasty invasive species, and prevent tremendous human suffering. Then again, it could have unintended social and ecological consequences—or be hijacked for malevolent purposes.
The concept of a gene drive has been around for decades. In a 2003 paper, the British geneticist Austin Burt—inspired by naturally occurring “selfish” genes that copy themselves around the genome with the aid of enzymes that cut the DNA at precise locations—suggested that harnessing this ability and improving upon it would allow scientists to engineer natural populations, with an eye, for instance, toward preventing the spread of malaria.
Burt’s insight wasn’t practical, though, prior to the fairly recent invention of a breakthrough technique called CRISPR-Cas9 gene editing. With this innovation, a scientist uses customized ribonucleic acid (RNA) guide sequences to deliver a molecular scissors (an enzyme called Cas9) to a precise spot on a chromosome. The enzyme snips the double helix, prompting the cell’s DNA-repair machinery to kick in and patch things up—and in the process replacing the regular “wild-type” gene at that location with a lab-engineered DNA sequence. (Here’s a simple diagram.)
One spring day in 2013, about a decade after Burt’s paper appeared, a 30-year-old researcher named Kevin Esvelt was out walking in the Boston-area greenbelt known as the Emerald Necklace, pondering his next move. Esvelt, a post-doctoral fellow working with the renowned Harvard geneticist George Church, had ruled out working on the development of new CRISPR techniques. “The field had become so crowded,” he recalls via email, “it seemed likely almost anything I tried would be pursued by at least three other labs.”

Esvelt in his current laboratory.
MIT Media Lab
As he walked along, Esvelt idly wondered whether any of the greenbelt’s wild creatures would end up being gene-edited in the decades to come. You could do it, of course, by introducing the CRISPR elements into wild-animal embryos. But why bother? The modified genes would become less and less prevalent with each generation of offspring. Natural selection would eventually weed them out of the population entirely.
And that’s when it hit him: Scientists had been putting the CRISPR tools into their target cells as separate pieces. What if you introduced them into the embryos as a single, heritable element? Those creatures and their descendants—all of them—would retain the gene-editing ability in their DNA. The system would be self-propagating. In short, you could rig nature’s game so your gene would win every time!
Esvelt was practically giddy with the possibilities. “The first day was total elation,” he told me. He found Burt’s paper and began fantasizing about all the lives gene drive might save. But the elation didn’t last long. A mistake—or a deliberate act—he soon realized, would alter an entire species. An experimental drive could escape into the wild before society agreed that it was okay. Perhaps gene drive could even be used as a weapon of sorts—a means for sowing havoc. “Once it hit me,” he recalls, “well, there was a flash of pure terror, followed by an obsessive evaluation of potential misuses.” Like Enrico Fermi, the scientist who demonstrated the first nuclear chain reaction back in 1942—Esvelt would be letting a very big cat out of the bag.
He took his ideas and concerns to his mentor, George Church. A scientist’s usual first instinct is to test an exciting hypothesis right away to see whether it’s viable, and then be the first to press with a blockbuster paper. This felt different. “We decided not to immediately test it in the lab—not because we couldn’t do it safely, but because we felt that no technology like this should be developed behind closed doors,” Esvelt says. “The question was whether it was safe to tell the world.” At Church’s urging, they brought on Jeantine Lunshof, an ethicist, and Ken Oye, a social scientist and policy expert: “Ken’s first words after I described the probable capabilities were not publishable.”
The researchers determined that their best course was to go public before doing any experiments. They solicited feedback from fellow molecular biologists, ecologists, risk analysts, public policy and national security experts, and representatives of environmental nonprofits. Only then, in July 2014, did they publish a pair of papers on gene drive’s uses and policy implications.
This summer, a group of researchers that consults for the federal government was tasked with analyzing the technique’s potential risks—including the possibility that it could be used for biowarfare. “The range of nefarious possibilities based on genetically engineered microorganisms is already vast,” Steven Block, an expert in bioterror defense at Stanford University, told me in an email. “The right question to ask is whether a hypothetical gene-drive-based bioweapon, which is based on multicellular organisms, would afford any specific advantages over something based on microorganisms. Would it be more powerful? Cheaper? Easier to construct? Would it be more accessible to an adversary? Would it afford any special ‘desirable’ properties as a weapon, from either a strategic or tactical perspective? I’d argue that, at least for the time being, gene drive seems to have done little to change the lay of the land.”
Accidents, mistakes, and unsanctioned releases are a separate concern. But Esvelt and his peers realized, to their great relief, that gene drives can be overwritten; they spread slowly enough through a population and are easy enough to detect, Esvelt says, that researchers should be able to stop a rogue drive using something called an “immunizing reversal drive” that can cut up the engineered sequence and restore the original genes. (He and Church have demonstrated the reversal process in yeast.) In any case, he says, it would be “difficult to imagine any possible combination of side-effects worse than a disease like malaria.”
Over the past couple of years, several labs have proved that gene drives work as hypothesized. The next step is to convince society they can be tested safely. Each drive is different, so potential risks and benefits have to be weighed on a case-by-case basis. But one big-picture problem is that wild creatures don’t respect human boundaries. A drive could easily scamper or fly or tunnel across borders and into areas where it hasn’t been sanctioned by local authorities. And that, Esvelt says, could trigger “international disputes or even wars.”
In his new position as head of the Sculpting Evolution group at the Massachusetts Institute of Technology’s Media Lab, Esvelt is working on gene-drive variations that can limit the spread of the engineered genes to a given number of generations. But diplomacy will be needed regardless. “For malaria, the case for an international agreement is obvious,” Esvelt says. Ditto the New World screwworm, whose “existence in the wild is an atrocity from an animal welfare perspective—it literally exists by eating higher mammals alive, causing excruciating agony.”
In 2015, Austin Burt and his collaborators unveiled a gene drive designed to decimate populations of the African malaria mosquito Anopheles gambiae by rendering all female offspring sterile, although for statistical reasons, it is “quite implausible” for a gene drive system to completely wipe out a problematic species, Esvelt says. “Suppress a population, sure. Locally eliminate, possibly. But extinction? Not by itself.”
Anthony James, a geneticist at the University of California-Irvine, opted to target the disease directly. In 2015, he and his colleagues lab-tested a drive that enlists a pair of synthetic antibodies to disable malaria in the gut of the South Asian mosquito Anopheles stephensi. The dual attack—which targets two distinct phases of the parasite’s life cycle—should be all but impossible for the organism to overcome. In the highly unlikely event that these antibodies were to get into another insect species, they shouldn’t cause any problems. And because the mosquito population remains intact, their predators won’t lack for food.
James says his malaria drive will be ready for field tests within two years—either in huge outdoor cages or within a naturally confined environment such as an island. But is humanity ready to allow it? “It’s all new stuff. This is the problem. There’s no pathway,” he says. Securing permission to move forward with testing will depend entirely on the local mood and regulatory situation. As for deploying gene drive on a species-wide scale? Esvelt is skeptical that nations would accept wild releases without constraints in place that would limit their scope.
One way or the other, something has to change on the mosquito front. Conventional control methods—monitoring and education, poisons, door-to-door efforts to eliminate standing water—aren’t working. Poor countries in particular lack the resources to keep the bugs at bay, and because insects and microorganisms evolve so rapidly, our chemical weapons are rapidly losing their effectiveness. According to Bill Reisen, a retired UC-Davis mosquito expert, California mosquitoes can now tolerate compounds from three major families of insecticides that were once used to kill them: “The opportunities for control are becoming progressively limited.” The Centers for Disease Control and Prevention reports that Plasmodium falciparum, the world’s deadliest malaria parasite, has developed resistance to “nearly all” antimalarial drugs.
A Zika vaccine seems to be on the horizon, but dengue remains a frustratingly elusive target for vaccine developers. UC-Davis geneticist Greg Lanzaro told me last year that, were it solely up to him, he would deploy gene drive as soon as scientifically feasible to beat back the Aedes mosquitoes that spread these diseases. Esvelt has heard similar sentiments from peers in several fields. “As a scientist, it’s hard to accept nontechnical limitations, especially when we could seemingly save so many lives if those constraints somehow magically vanished,” he says. “But they won’t.”
One thing is for sure: “The first effort has to be an unqualified success,” James says. “If there’s a trial and it’s a disaster—meaning it doesn’t prevent an epidemic—the technology is going to be set back.” Esvelt points to Jesse Gelsinger, an 18-year-old whose death during a 1999 gene therapy trial stifled progress in that field for a decade or more. “An accident involving a CRISPR gene drive—which would be viewed as reckless scientists accidentally turning an entire species into GMOs—would almost certainly have similar effects,” he says. And in the case of malaria, the delay “would likely result in the otherwise preventable deaths of millions of children.”
So he’s willing to wait to get it right. Indeed, in Esvelt’s view, gene drive is so existentially powerful that it demands a new era of scientific transparency. If researchers don’t rethink their longtime custom of competing behind closed doors, “we are likely to open extremely dangerous technological boxes without even realizing it.”
A deeply collaborative approach with preregistered experiments, Esvelt says, would help scientists identify unforeseen dangers and ensure that those “boxes remain closed until we can develop countermeasures.” Such a radical departure from the current culture of secrecy would require nothing short of a sea change in the scientific community. But it might be worth the effort. As Esvelt puts it, “The greatest potential application of gene drive is to engineer the scientific ecosystem.”
This story has been corrected to more accurately describe when the concept of gene drive originated.